As in previous years, stopping TKI treatment was a widely discussed topic at this year’s ASH meeting. New data on trials like EURO-SKI, DASFREE and others are underlining the findings of previous trials and are increasing the certainty on the safety of stopping treatment in a very controlled, closely monitored clinical setting. There is still uncertainty about factors that might predict whether a patient can successfully stop treatment or will experience a recurrence of the disease leading to re-starting therapy. There is still no clarity about the underlying biological mechanisms why some patients can stop successfully while others can’t.
Ongoing trials like EURO-SKI will provide a lot of answers, but because there is a lot of demand for stopping treatment while there is only a small number of trials that are still recruiting, stopping treatment is happening a lot “out there in the field”. As demonstrated by data presented data at the ASCO conference in June 2017, this may lead also to bad clinical practice when the requirements and potential risks are not taken serious. To provide more certainty on the requirements, first official clinical recommendations are emerging, but they are based on early data. We’ve tried to summarize what we heard and learned at ASH and what current recommendations say.
EURO-SKI: The longer in deep molecular response (MR4), the higher the chance for treatment-free remission
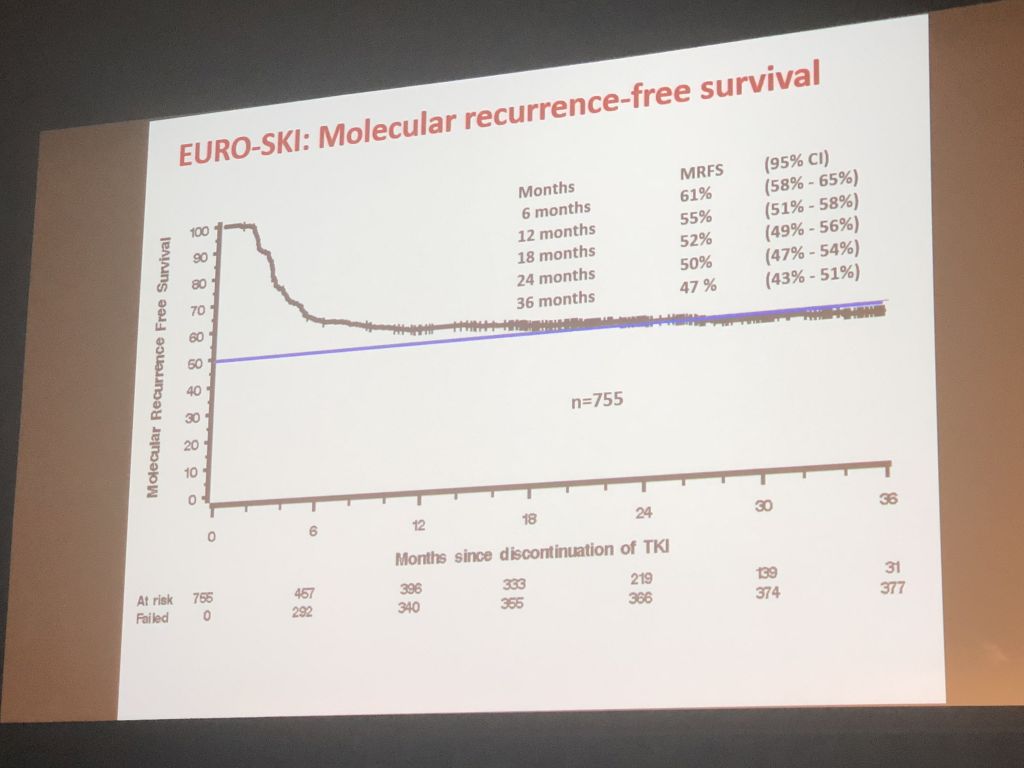
Susanne Saußele presented data of the EURO-SKI study, the largest study on stopping treatment which has recruited 868 patients that have stopped any TKI treatment in deep molecular response.
Across all STOP studies, more than 2500 patients have stopped TKI treatment within those studies. Therapy-free remission is achievable for 40-55% of patients after they have stopped TKI treatment in deep molecular response (at least MR4, BCR-ABL below 0.01%) following several years of TKI treatment. Around 9 of 10 patients who fail to stop treatment successfully lose their MMR already within 6 months after cessation of treatment. Current trials are trying to identify prognostic factors that allow to predict the likelihood that an individual patient will remain in remission after cessation of treatment.
Prof Saußele presented the latest analysis of the data of EURO-SKI. In this independent academic study, patients had to be in treatment with any TKI for at least 3 years and be in a deep molecular response (MR4) for at least 1 year to be eligible to stop treatment. Patients had to restart treatment if their PCR rose above MMR (BCR-ABL 0.1%). 868 patients have been recruited. 47% of patients remained in therapy-free remission at 36 months after stopping treatment. Even though 9 of 10 recurrences happened within the first six months after the stop, there was a slow but steady decline of patients in therapy-free remission afterwards – there have been late relapses after up to 36 months. In the discussion, one Scandinavian doctor even mentioned a late recurrence of one of his stop patients after 5 years. This means – PCR monitoring will most likely be required forever.
 The prognostic modelling of EURO-SKI could so far be conducted on the data of 448 patients. The risk score at the time of diagnosis (e.g. SOKAL score), the type of TKI used, the depth of molecular response below MR4 did not have any influence on the success rate. A patient’s prior history of suboptimal response or resistance to TKI therapy decreased probability for therapy-free remission. Most importantly, the duration of treatment and the duration of deep molecular response prior to stopping treatment influenced the success rate: the longer the TKI treatment and MR4 remission, the higher the probability to stay therapy-free. Interestingly, the probability to stay in remission after stopping increased by 3% for every year in MR4 remission, while the overall time on TKI treatment had a much lower influence (1% per year). Best results of stopping Imatinib were achieved if patients had at least 5.8 years of Imatinib therapy and at least 3.1 years of deep molecular response – similar data for Dasatinib and Nilotinib has not yet been analyzed. Additionally, a patient’s prior history of suboptimal response or resistance to TKI therapy also decreased probability for therapy-free remission.
The prognostic modelling of EURO-SKI could so far be conducted on the data of 448 patients. The risk score at the time of diagnosis (e.g. SOKAL score), the type of TKI used, the depth of molecular response below MR4 did not have any influence on the success rate. A patient’s prior history of suboptimal response or resistance to TKI therapy decreased probability for therapy-free remission. Most importantly, the duration of treatment and the duration of deep molecular response prior to stopping treatment influenced the success rate: the longer the TKI treatment and MR4 remission, the higher the probability to stay therapy-free. Interestingly, the probability to stay in remission after stopping increased by 3% for every year in MR4 remission, while the overall time on TKI treatment had a much lower influence (1% per year). Best results of stopping Imatinib were achieved if patients had at least 5.8 years of Imatinib therapy and at least 3.1 years of deep molecular response – similar data for Dasatinib and Nilotinib has not yet been analyzed. Additionally, a patient’s prior history of suboptimal response or resistance to TKI therapy also decreased probability for therapy-free remission.
Stopping Dasatinib: the DASFREE study
 Dr Neil Shah presented the 1-year follow-up data of the Dasatinib stop study DASFREE where Dasatinib was stopped in deep molecular response MR4.5 (BCR-ABL 0.0032%, so more strict than EURO-SKI). Patients needed to have received Dasatinib for at least 2 years (so one year less than in EURO-SKI) and a deep molecular response for at least 1 year (same as in EURO-SKI). Patients also had to restart treatment at the loss of MMR.
Dr Neil Shah presented the 1-year follow-up data of the Dasatinib stop study DASFREE where Dasatinib was stopped in deep molecular response MR4.5 (BCR-ABL 0.0032%, so more strict than EURO-SKI). Patients needed to have received Dasatinib for at least 2 years (so one year less than in EURO-SKI) and a deep molecular response for at least 1 year (same as in EURO-SKI). Patients also had to restart treatment at the loss of MMR.
44% had received Dasatinib first line, all other patients had received it after resistance or intolerance to prior treatment. The TFR rate at 1 year was at 48% — 54% for first-line patients and 43% for second and subsequent lines of treatment. Median duration from discontinuation of therapy to loss of MMR was 4 months (range 1-16 months). All patients who had to restart treatment quickly regained MMR after therapy was reinitiated (median time to regain MMR was 1.9 months), 90% regained at least MR4.5. In terms of prognostic factors, age, sex, duration of prior TKI, prior therapy line, Sokal score were all not influencing success of stopping. 9.5% of patients had shown withdrawal symptoms, and most events resolved without medical intervention.
Dr Shah concluded that the DASFREE study strongly supports the feasibility of stopping treatment in deep molecular response in first line treatment with Dasatinib and beyond.
 8-year follow-up data of the TWISTER STOP study: In patients with long-term therapy-free remission, number of residual CML cells may further decline
8-year follow-up data of the TWISTER STOP study: In patients with long-term therapy-free remission, number of residual CML cells may further decline
On a poster, Dr Tim Hughes from Australia presented the long-term follow-up data of the TWISTER study which enrolled 40 imatinib-treated patients in the 2006. As reported in earlier years, 42.7% of patients remained in therapy-free remission for a median of 8.6 years (range 5.8-10.8 years). This year’s poster presented the dynamic of minimal residual disease over time in 6 patients in TFR, using the highly sensitive gDNA PCR, which is capable to detect BCR-ABL up to a level of MR6. In those patients, the residual disease level decreased from a median of MR5.3 to a median of MR6 in the most recent three years, so there was a reduction in leukemic cells even after the cessation of Imatinib treatment. The authors suggest that this could be either due to extinction of long-lived CML cells that lack the capability to self-renew, or the depletion of slowly proliferating CML precursor cells.
Recommendations on stopping TKIs outside of a clinical trials
The community of CML experts is obviously still debating the different institutional requirements and individual patient criteria to stop CML treatment in a safe manner outside of clinical trials.
The US-American NCCN Guideline on CML, published in early 2017, provides guidance on stopping treatment, but there is no PCR standardisation in the USA. An update to the CML Treatment Recommendations of the European LeukemiaNET is still work in progress, but has not yet been published. The European ESMO CML Guideline 2017 which also provide recommendations on stopping treatment has been published in Summer 2017. In addition, in the ASH Education Session, Francois-Xavier Mahon presented personal recommendations on stopping treatment outside of clinical trials.
There is agreement between all recommendations that treatment discontinuation may be considered in individual patients if proper, high-quality, standardized monitoring can be ensured. Additionally, institutional requirements of the clinic and lab in terms of safe supervision and regular follow-up of the patient, quantifiable BCR–ABL transcripts, availability of standardiszed PCR with 4.5log sensitivity, and the capacity of quick turnaround time of PCR results, as well as informing the patient about the risk of stopping failure and about the need of frequent monitoring, should be provided. The recommendations however differ in terms of number of years of TKI therapy (3-5 years), the depth of molecular response (MR4 or MR4.5) and the number of years in deep molecular response (2-3 years) before considering to stop treatment. There are also gaps between the different recommendations.
To provide an overview of the different recommendations on minimal requirements when considering treatment-free remission, we have summarized them below:
| Patient characteristics | NCCN Guidelines Version 2.2017 CML | ESMO Clinical Practice
Guidelines (8/2017) |
FX Mahon Personal Guidance (ASH 2017) |
| Minimum age | >18 | Not defined | Not defined |
| CML risk score | Not defined | Low or intermediate risk | Not defined |
| CML phase | Chronic phase CML, no prior history of advanced/blast phase | Chronic phase CML. Optimal response level to first-line therapy. | Chronic Phase CML, no patient history of resistance to treatment |
| BCR-ABL transcript | Quantifiable BCR-ABL transcript | Typical b2a2- or b3a2-BCR–ABL transcripts, or atypical transcripts
which can be quantified over a 4.5 log dynamic range |
Not defined |
| TKI therapy duration before stopping | >= 3 years | >= 5 years | >= 5 years |
| Response level before stopping | MR4 >= 2 years | MR4.5 reached, but
MR4 >= 2 years |
Three points of BCR-ABL level per year mandatory with MR4 during 3 following years or MR4.5 during 2 following years (1 point in MR4 could be acceptable) |
| Restart criteria | Loss of MMR | Loss of MMR | Loss of MMR. use same TKI in the following month after loss of MMR. |
| Institutional requirements | NCCN Guidelines Version 2.2017 CML | ESMO Clinical Practice
Guidelines (8/2017) |
FX Mahon Personal Guidance (ASH 2017) |
| Clinical requirements | CML speciality center | Institution that has structured follow-up to enable rapid intervention in rising BCR-ABL | Not mentioned |
| Lab requirements | Reliable PCR with 4.5log sensitivity (IS) and provides results in 2 weeks | High quality internationally standardised, accurate,
sensitive qRT-PCR with 4.5log sensitivity that provides results within 4 weeks and has capacity to perform PCR every 4-6 weeks |
Certified laboratory with >4log sensitivity |
| Monitoring schedule after stopping | Month 1-6: monthly
Month 7-24: every two months, 3-monthly later on when in MMR |
Month 1-6: monthly, Month 6-12: 6-weekly, 3-monthly later on |
Not defined |
| TFR failure monitoring schedule | Month 0-6: monthly.
Every three months thereafter infinitely. Molecular testing if MMR is not regained after 6 months. |
Not defined | Not defined |
| Other | Monitoring of withdrawal symptoms. Reporting of progression to advanced phases to NCCN CML panel. | – | Declaration to an international or national registry. |